Introduction Treatment of relapsed/refractory acute lymphoblastic leukemia (r/r ALL) remains challenging in spite of recent progress with immunotherapy and targeted small-molecule combinations. Growing awareness of the molecular complexity of the disease with subtype-specific cancer dependencies positions functional drug testing as a promising precision tool to improve the prediction of clinical drug efficacy and adapt treatment. We leverage automated, image-based Drug Response Profiling (DRP) and machine learning to deliver individualized therapeutic options in real time for ALL patients with urgent medical needs. Here we report on the DRP experience of 307 primary samples from 278 patients with BCP-ALL, T-ALL/T-LBL and mixed phenotypic acute leukemia (MPAL) registered from 2016 to 2022 in a non-interventional setting.
Methods We have generated a reference set of 141 diagnostic samples from patients with high-risk (75%) or standard/medium-risk (25%) disease as defined by the clinical trial AIEOP-BFM ALL 2017, alongside 122 samples with first and 44 with more advanced relapse. We assayed cell viability at single-cell level from viably frozen bone marrow (BM) samples, co-cultured with hTERT-immortalized mesenchymal stromal cells (MSC) and following a 72h drug incubation with a library of up to 110 compounds. We quantified cell counts at five concentrations in duplicates by segmenting and classifying MSC, live and dead leukemia cells using a modular image processing pipeline deployed on cloud infrastructure. We longitudinally confirmed assay consistency with PDX models and monitored integrity of the stromal layer on a per-sample basis. In a subset of cases (15-20%), relevant stromal detachment or low leukemia survival led to samples not meeting all quality control standards. Drugs were ranked by the area under the dose response curve (AUC) and referenced to all previous samples. This patient-specific ex vivo drug fingerprint was reported to the treating physician with a typical turn-around time of less than two weeks.
Results Dexamethasone sensitivities measured ex vivo in BM samples from pediatric front-line patients, who were treated on a one-week cytoreductive steroid pre-phase followed by a four-drug induction regimen, correlated significantly with clinical responses at d8 (PGR vs. PPR, p<0.001), d15 (flow MRD below 10%, p<0.001) and d33 (PCR MRD below E-2, p<0.001).
By integrating dose-response profiles from all patients, we observed differential drug activities across cytogenetic groups and identified exceptional responders to Venetoclax in very high-risk subtypes including TCF3::HLF (5 patients, AUC percent rank: 0.03 - 0.19) as well as refractory T-ALL.
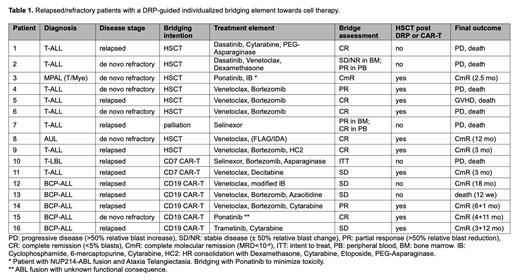
In 77 r/r T-ALL patients from 12 different countries, DRP supported selection of a personalized bridging regimen prior to consolidation with cellular therapy. Top-scoring drug sensitivities (10th percentile of AUC) were found for a variety of agents including Imatinib, Dasatinib, Venetoclax, Bortezomib, Selinexor and different nucleoside analogs. Eleven T-ALL and 5 BCP-ALL patients received a DRP-guided bridging element involving compounds with high ex vivo drug efficacy and complemented by chemotherapy (Table 1). The other 66 r/r patients were treated according to physician's choice incorporating information on drug resistance detected by DRP in real time. Bridging strategies implementing DRP-guided therapy blocks stabilized or reduced disease burden in 15/16 patients allowing HSCT or infusion of CAR-T cells in 12 patients with 8 patients still being in remission at last follow-up.
Conclusion We describe an array of pharmacological sensitivity and resistance patterns across a heterogeneous, high-risk cohort of BCP- and T-ALL patients. We identified individual susceptibilities to targeted agents of different classes including BH3-mimetics, proteasome, tyrosine kinase and exportin inhibitors as well as standard-of-care chemotherapeutics. Prospective recording of drug response profiles offers opportunities to systematically correlate ex vivo and clinical responses and inform personalized therapies. These data strongly advocate inclusion of DRP into randomized, early and late phase clinical trials for high-risk pediatric ALL, comprehensively integrating molecular and functional precision information to improve patient stratification and long-term outcome after HSCT and CAR-T.
Disclosures
Balduzzi:Novartis, Amgen, Medac, Neovii: Speakers Bureau. Elitzur:Medison Pharma: Honoraria; Jazz Pharmaceuticals: Honoraria. Attarbaschi:JazzPharma: Honoraria. Ansari:Jazz Pharmaceutical: Other: traveling grant and presentation inside the company on HSCT; NovoNordisk: Other: traveling grant. Baruchel:Novartis: Honoraria; Celgene: Honoraria; Servier: Honoraria; Jazz: Honoraria; Astra-Zeneca: Honoraria. Schmiegelow:Novo Nordisk Foundation: Current holder of stock options in a privately-held company, Research Funding; Illumina, Jazz Pharmaceuticals, Servier, Amgen, Medscape: Honoraria. Cario:JazzPharma: Speakers Bureau; Servier, Amgen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal